Nuvaxovid
The Novavax COVID-19 vaccine sold under the brand names Nuvaxovid and Covovax among others is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI. Qualitative and quantitative composition.
Fda To Authorize Novavax S Covid 19 Vaccine Politico
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes.

. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. This is a multidose vial. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.
As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus. Nuvaxovid-rokote sopii lähes kaikille aikuisille. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further.
Nuvaxovid contains a version of a protein found on the. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. On December 20 2021 the.
2 days agoPublicerad idag 0702. The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. On August 19 2022 the Food and Drug Administration FDA authorized the Novavax COVID-19 Vaccine.
About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Nuvaxovid dispersion for injection. COVID-19 Vaccine recombinant adjuvanted 2.
Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Company Novavax should not be given to individuals. Nuvaxovid vaccine pause for young people justice system spending Västerås shooting young women have more debt than 10 years ago.
Full results from Nuvaxovids pivotal phase III trial were published in December 2021. Nuvaxovid contains a version of a protein found on the. Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older.
HSAs assessment is that although the. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. Det eftersom att data från.
This will enable us to start offering the Nuvaxovid. As such HSA will be monitoring the incidence rate of pericarditis or inflammation of the outer lining of the heart and myocarditis. Name of the medicinal product.
Rokotteesta ei myöskään ole haittaa vaikka. Novavax Announces Shipments Of Its. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
Nuvaxovid contains a version of a protein found on the. The Summary of Product Characteristics is a description of a. Beslutet är temporärt och gäller från.
The first batch of Nuvaxovid is expected to arrive in.
Novavax Nuvaxovid Gets Expanded Conditional Marketing Authorization In Eu For Use As Booster For Adults Aged

Nuvaxovid Archives Drug Discovery And Development
Switzerland S Federal Office Of Public Health Recommended Nuvaxovid Nvx Cov2373 As A Heterologous And Homologous Booster For Immunization To Prevent Covid 19 Caused By Severe Acute Respiratory Syndrome Novavax

Novavax S Covid 19 Vaccine Approved For Canadians 18 And Older Cbc News
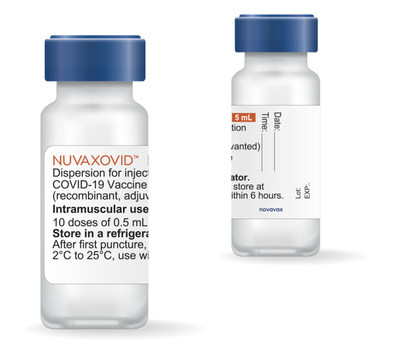
Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022
New Protein Based Covid 19 Vaccine Could Help Boost Rates Say Pharmacists Cbc News

Vaccine Against Coronavirus Nuvaxovid Novavax Niph
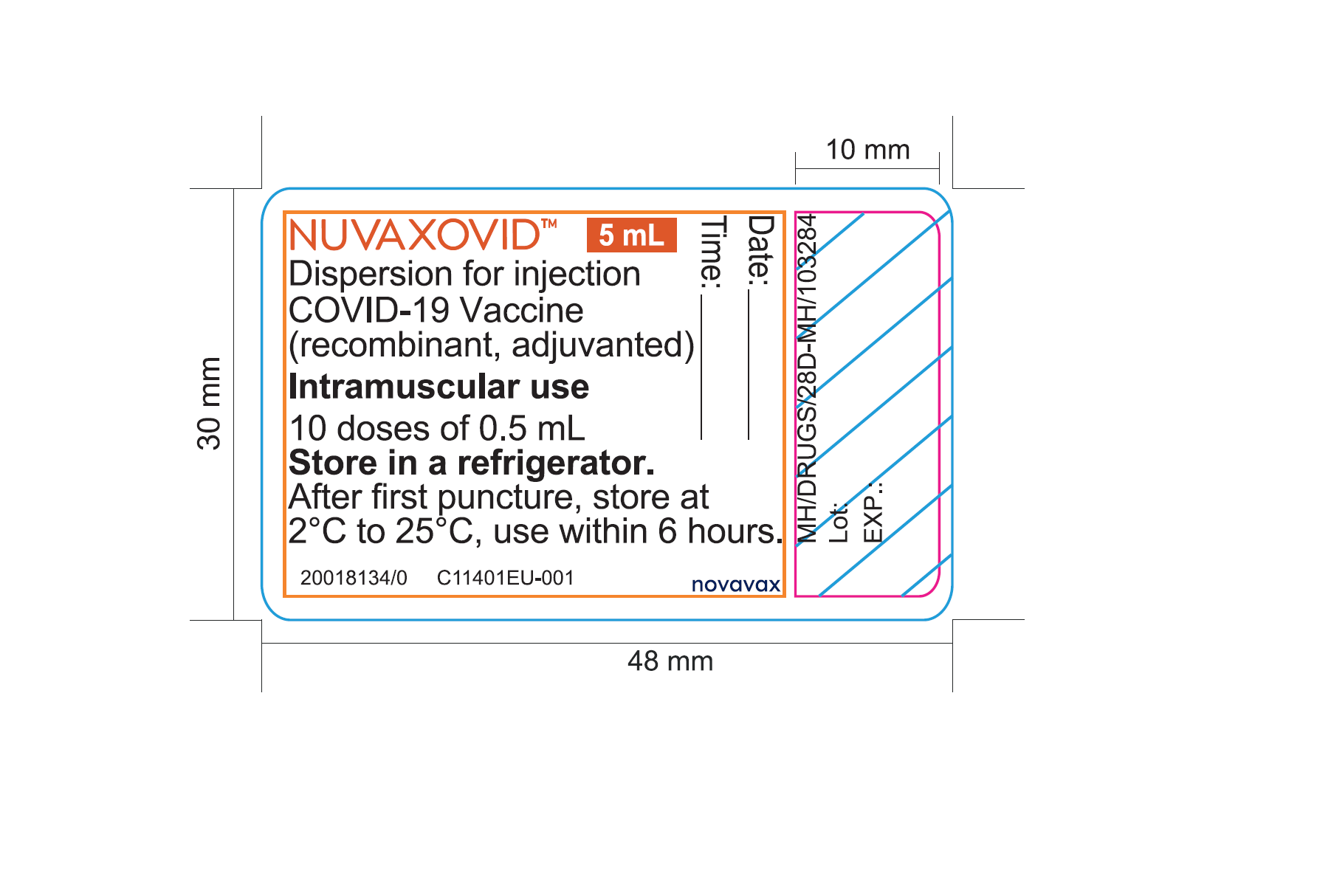
Distribution Of Nuvaxovid With English Only Vial And Carton Labels Canada Ca

Novavax Nuvaxovid Covid 19 Vaccine Star Pharmacy

Infomesen Long Covid 19 Nuvaxovid Novavax Vaksin Australian Government Department Of Health And Aged Care

Nuvaxovid Covovax Novavax Vaccine Covid 19 Info Vaccines

Novavax Covid Vaccine Efficacy In Omicron Delta Variants Among Unknowns Ahead Of Advisory Cmte Pink Sheet

Msf Canada On Twitter Msf Comment On Canadian Approval Of The Novavax Nuvaxovid Vaccine For Covid19 And Canada S Role In Global Vaccineequity Https T Co Vkdoxh9sjy Twitter
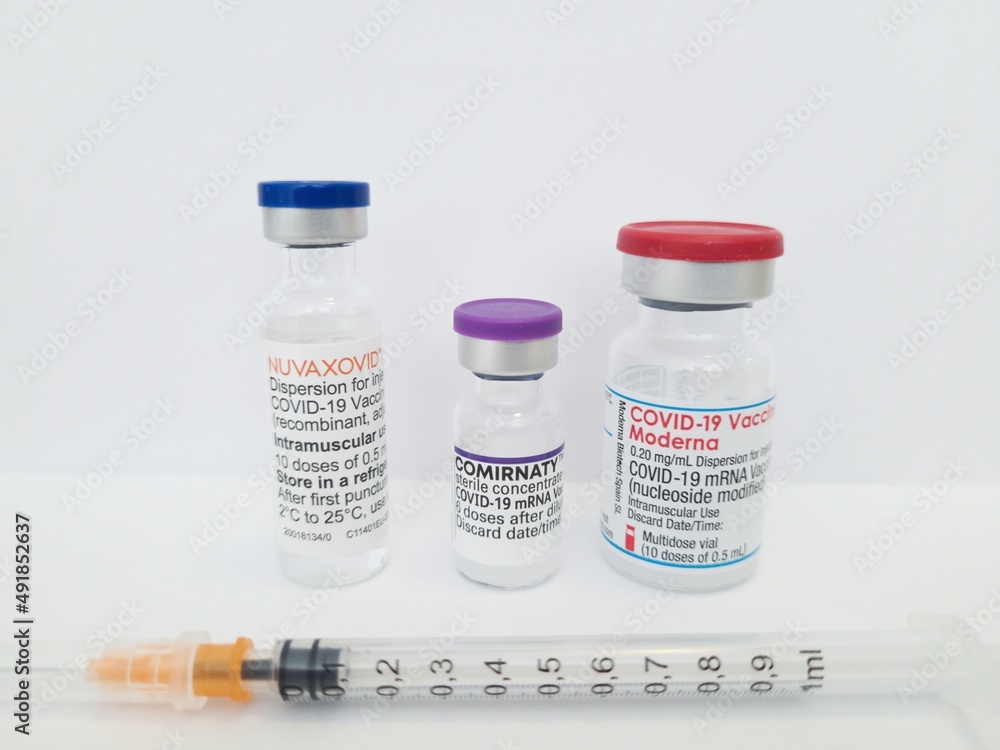
Biontech Comirnaty Moderna Covid 19 Vaccine And Novavax Nuvaxovid Vaccine In A Vial With A Syringe Stock Photo Adobe Stock

Novavax Nuvaxovid Covid 19 Vaccine Granted Expanded Conditional Marketing Authorization In The European Union For Use As A Booster For Adults Aged 18 And Older Sep 12 2022

Fda Advisers Overwhelmingly Endorse Novavax Covid 19 Vaccine Ars Technica
Switzerland Approves Novavax S Covid Vaccine For 12 18 Year Olds Swi Swissinfo Ch

Novavax Covid 19 Vaccine To Be Rolled Out In Australia From Next Month
